The Food Safety and Standards Authority of India (FSSAI) has launched a surveillance drive to curb the menace of spurious drugs manufactured by nutraceutical companies operating across the country. As part of this initiative, the regulatory authority has initiated its first set of drives in Himachal Pradesh, directing its Regional Office, North, to take immediate action against the defaulting Food Business Operators (FBOs) involved in the production of spurious drugs.As part of this drive, 21 facilities operating in Baddi, Himachal Pradesh, were inspected and 111 samples have been lifted during 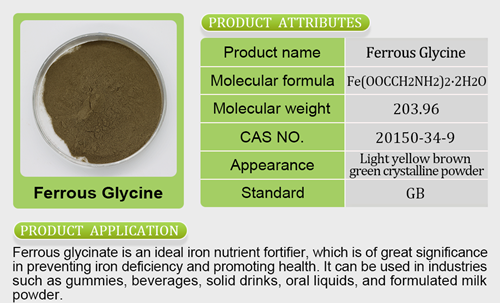 June 7-9, 2023. Further, 25-30 per cent of the nutraceuticals manufacturing facilities in Himachal Pradesh will be inspected by the end of June 2023. To address this issue, the CEO, FSSAI, convened a meeting with major manufacturers of health supplements and nutraceuticals of Baddi, Himachal Pradesh, on June 20, 2023, at FSSAI headquarters.The Food Safety and Standards Authority of India (FSSAI) has launched a surveillance drive to curb the menace of spurious drugs manufactured by nutraceutical companiemagnesium citrate benefitss operating acitracal slow release 1200 costcocross the country. As part of this initiative, the regulatory authority has initiated its first set of drives in Himachal Pradesh, diferric pyrophosphate hs coderecting its Regional Office, North, to take immediate action against the defaulting Food Business Operators (FBOs) involved in the production of spurious drugs.During the meeting, the CEO, FSSAI issued a stern warning, emphasising the absolute necessity for strict compliance with nutraceutical regulations. Non-compliance was highlighted as havi
June 7-9, 2023. Further, 25-30 per cent of the nutraceuticals manufacturing facilities in Himachal Pradesh will be inspected by the end of June 2023. To address this issue, the CEO, FSSAI, convened a meeting with major manufacturers of health supplements and nutraceuticals of Baddi, Himachal Pradesh, on June 20, 2023, at FSSAI headquarters.The Food Safety and Standards Authority of India (FSSAI) has launched a surveillance drive to curb the menace of spurious drugs manufactured by nutraceutical companiemagnesium citrate benefitss operating acitracal slow release 1200 costcocross the country. As part of this initiative, the regulatory authority has initiated its first set of drives in Himachal Pradesh, diferric pyrophosphate hs coderecting its Regional Office, North, to take immediate action against the defaulting Food Business Operators (FBOs) involved in the production of spurious drugs.During the meeting, the CEO, FSSAI issued a stern warning, emphasising the absolute necessity for strict compliance with nutraceutical regulations. Non-compliance was highlighted as havi ng severe consequences, including the possibility of licence suspension or cancellation, as well as the initiation of criminal cases.The Food Safety and Standards Authority of India (FSSAI) has launcmagnesium glycinate tablets holland and barretthed a surveillance drive to curb the menace of spurious drugs manufactured by nutraceutical companies operating across the country. As part of this initiative, the regulatory authority has initiated its first set of drives in Himachal Pradesh, directing its Regional Office, North, to take immediate action against the defaulting Food Business Operators (FBOs) involved in the production of spurious drugs.Recognising the significance of a coordinated effort, the FSSAI has directed the Commissioner of Food Safety, Himachal Pradesh, to provide full supferrous sulfate while pregnantport in carrying out the surveillance drive effectively. FBOs found to be in violation may be prosecuted under Section 59 of FSS Act, 2006, where punishment like lifetime imprisonmen
ng severe consequences, including the possibility of licence suspension or cancellation, as well as the initiation of criminal cases.The Food Safety and Standards Authority of India (FSSAI) has launcmagnesium glycinate tablets holland and barretthed a surveillance drive to curb the menace of spurious drugs manufactured by nutraceutical companies operating across the country. As part of this initiative, the regulatory authority has initiated its first set of drives in Himachal Pradesh, directing its Regional Office, North, to take immediate action against the defaulting Food Business Operators (FBOs) involved in the production of spurious drugs.Recognising the significance of a coordinated effort, the FSSAI has directed the Commissioner of Food Safety, Himachal Pradesh, to provide full supferrous sulfate while pregnantport in carrying out the surveillance drive effectively. FBOs found to be in violation may be prosecuted under Section 59 of FSS Act, 2006, where punishment like lifetime imprisonmen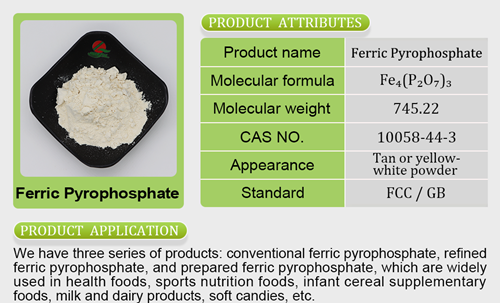 t or a fine of not less than Rs 10 lakh will be imposed.
t or a fine of not less than Rs 10 lakh will be imposed.

FSSAI Warns Nutraceutical Companies in Himachal Pradesh
Search
Get In Touch
Please feel free to leave a message. We will reply you in 24 hours.
Product categ
- Custom Series9 products
- Granulation Series5 products
- Microencapsulated Series2 products
- Supermicro Series2 products
- Mineral Nutrients26 products
- Calcium Salt6 products
- Copper Salt1 product
- Iron Salt7 products
- Magnesium Salt3 products
- Manganese Salt1 product
- Potassium Salt3 products
- Sodium Salt2 products
- Zinc Salt3 products
- Premix4 products
- Mineral Premix2 products
- Vitamin Premix2 products



