The Food Safety and Standards Authority of  India (FSSAI) has notified regulations that prohibit sale of food for infant nutrition except under the BIS certification mark.The regulations were n
India (FSSAI) has notified regulations that prohibit sale of food for infant nutrition except under the BIS certification mark.The regulations were n otified as Food Safety and Standards (Prohibition and Restriction on Sales) First Amendment Regulations, 2022, and shall come into force from April 1, 2023.The Food Safety and Standards Authority of Indi
otified as Food Safety and Standards (Prohibition and Restriction on Sales) First Amendment Regulations, 2022, and shall come into force from April 1, 2023.The Food Safety and Standards Authority of Indi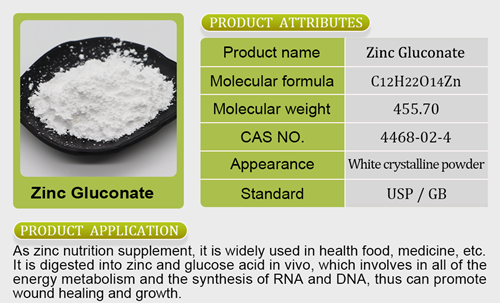 a (FSSAI) has notified regulations that prohibit sale of food for infant nutrition excliquid magnesium malate and glycinate отзывыept under the BIS certification maglycinate or malaterk.The regulations read,”No person shall manufacture, sell, store or exhibit for sale food for infant nutrition, except under Bureau of Indian Standards (BIS) Certification Mark, wherever BIS standards are available.”The Food Safety and Standards Authority of India (FSSAI) has notified regulations that prohibit sale of food for infant nutrition except under the BIS certification mark.The draft was made available in September 2021 and after receiving objections and suggestions from the public, the draft was considered by the FSSAI for final notification.The Food Safety and Standards Authority of India (FSSAI) has notified regulations that prohibit sale of food for infant nutrition except under the BIS certification mark.According to FSactive iron vs ferrous sulfateSAI, food for infant nutrition includes food for infants based on traditional food ingredients, food citracal d3 maximumfor special zinc gluconate anosmiamedicinal purposes intended for infants, follow up formula, infant food, infant milk substitute, infant formula, milk cereal based complementary food and processed cereal based complementary food.
a (FSSAI) has notified regulations that prohibit sale of food for infant nutrition excliquid magnesium malate and glycinate отзывыept under the BIS certification maglycinate or malaterk.The regulations read,”No person shall manufacture, sell, store or exhibit for sale food for infant nutrition, except under Bureau of Indian Standards (BIS) Certification Mark, wherever BIS standards are available.”The Food Safety and Standards Authority of India (FSSAI) has notified regulations that prohibit sale of food for infant nutrition except under the BIS certification mark.The draft was made available in September 2021 and after receiving objections and suggestions from the public, the draft was considered by the FSSAI for final notification.The Food Safety and Standards Authority of India (FSSAI) has notified regulations that prohibit sale of food for infant nutrition except under the BIS certification mark.According to FSactive iron vs ferrous sulfateSAI, food for infant nutrition includes food for infants based on traditional food ingredients, food citracal d3 maximumfor special zinc gluconate anosmiamedicinal purposes intended for infants, follow up formula, infant food, infant milk substitute, infant formula, milk cereal based complementary food and processed cereal based complementary food.

FSSAI Notifies Norms Prohibiting Sale of Infant Nutrition food Sans BIS Certification
Search
Get In Touch
Please feel free to leave a message. We will reply you in 24 hours.
Product categ
- Custom Series9 products
- Granulation Series5 products
- Microencapsulated Series2 products
- Supermicro Series2 products
- Mineral Nutrients26 products
- Calcium Salt6 products
- Copper Salt1 product
- Iron Salt7 products
- Magnesium Salt3 products
- Manganese Salt1 product
- Potassium Salt3 products
- Sodium Salt2 products
- Zinc Salt3 products
- Premix4 products
- Mineral Premix2 products
- Vitamin Premix2 products



