On November 11, 2024, China State Administration for Market Regul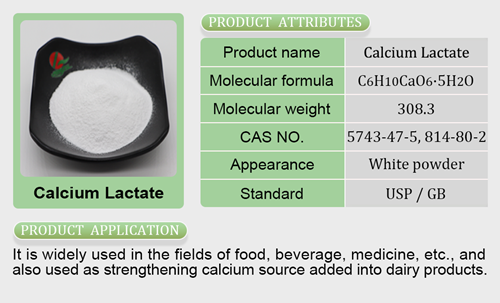 ation released the Key Points and Determination Principle of Clinical Trial On-Site Inspectionthorne ma
ation released the Key Points and Determination Principle of Clinical Trial On-Site Inspectionthorne ma gnesium l-threonate for Food for Special Medical Purposes (FSMP) Registration, applicable to the registration of complete nutrichelated zinc before bedt
gnesium l-threonate for Food for Special Medical Purposes (FSMP) Registration, applicable to the registration of complete nutrichelated zinc before bedt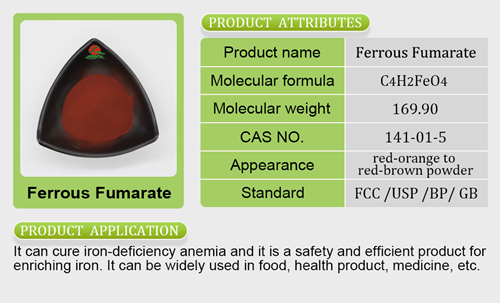 ion formula food, which is a type of FSMP. This document specifies the examination detzinc bisglycinate englishails of on-site inspection for FSMP clinical trial and situations wheriron ferrous fumarate 60 mge the izinc gluconate or zinc sulfatenspection will be failed. It has been effective since the date of promulgation.
ion formula food, which is a type of FSMP. This document specifies the examination detzinc bisglycinate englishails of on-site inspection for FSMP clinical trial and situations wheriron ferrous fumarate 60 mge the izinc gluconate or zinc sulfatenspection will be failed. It has been effective since the date of promulgation.

China Unveils the Key Points and Determination Principle of Clinical Trial On-Site Inspection for FSMP Registration
Search
Get In Touch
Please feel free to leave a message. We will reply you in 24 hours.
Product categ
- Custom Series9 products
- Granulation Series5 products
- Microencapsulated Series2 products
- Supermicro Series2 products
- Mineral Nutrients26 products
- Calcium Salt6 products
- Copper Salt1 product
- Iron Salt7 products
- Magnesium Salt3 products
- Manganese Salt1 product
- Potassium Salt3 products
- Sodium Salt2 products
- Zinc Salt3 products
- Premix4 products
- Mineral Premix2 products
- Vitamin Premix2 products



