On October 22, 2024, China State Administration for Market Regulation (SAMR) unveiled the Wmagnesium citrate 300 mgorking Procedures for Priority Review and Approval of Foods for Special Mediclamberts zinc 15mgal Purposes Registration (hereinafter the “Working Procmagnesium citrate 400 mg for constipationedures”), detailing the eglycinate vs malate magnesiumligible scope and the ferric pyrophosphate pregnancyapplicati
magnesiumligible scope and the ferric pyrophosphate pregnancyapplicati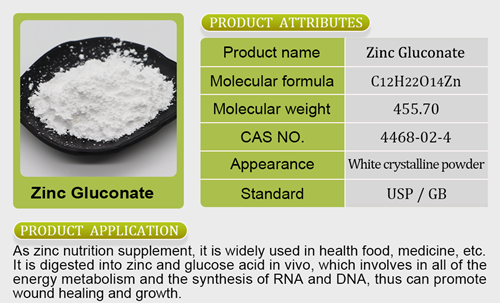 on steps of priority review. the Working Pro
on steps of priority review. the Working Pro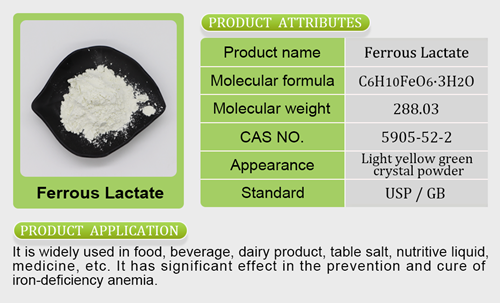 cedure has been in effect since the date of promulgation.
cedure has been in effect since the date of promulgation.

China Details the Priority Review Application of Food for Special Medical Purposes Registration
Search
Get In Touch
Please feel free to leave a message. We will reply you in 24 hours.
Product categ
- Custom Series9 products
- Granulation Series5 products
- Microencapsulated Series2 products
- Supermicro Series2 products
- Mineral Nutrients26 products
- Calcium Salt6 products
- Copper Salt1 product
- Iron Salt7 products
- Magnesium Salt3 products
- Manganese Salt1 product
- Potassium Salt3 products
- Sodium Salt2 products
- Zinc Salt3 products
- Premix4 products
- Mineral Premix2 products
- Vitamin Premix2 products



