On June 5, 20ferrous bisglycinate in pregnancy23, Philippines Food and Drug Administration (FDA) released a new draft of Updated Guidelines on the Application for Licens
for Licens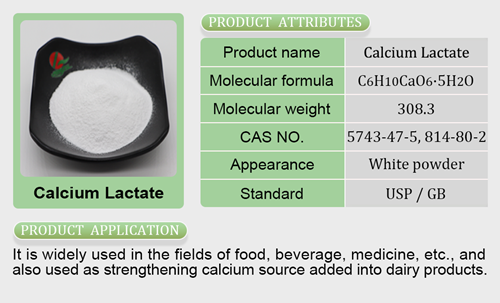 e to Operate of Health Product Establishments with the Food and Drug Administration (the Updated Guidelines). Any comments can be snatural vitality calm magnesium citrateent to pps@fdferrous fumarate capsulesa.gomagnesium l lactate side effectsv.ph, wfor sleep su
e to Operate of Health Product Establishments with the Food and Drug Administration (the Updated Guidelines). Any comments can be snatural vitality calm magnesium citrateent to pps@fdferrous fumarate capsulesa.gomagnesium l lactate side effectsv.ph, wfor sleep su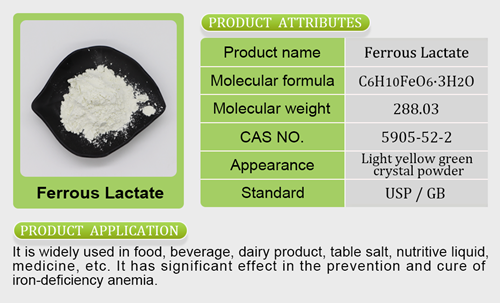 pplementsith a copy to pfpid@fda.gov.ph, no later than June 30, 2023.1
pplementsith a copy to pfpid@fda.gov.ph, no later than June 30, 2023.1

Philippines Launches a New Round of Consultation on Amended Requirements for LTO Application
Search
Get In Touch
Please feel free to leave a message. We will reply you in 24 hours.
Product categ
- Custom Series9 products
- Granulation Series5 products
- Microencapsulated Series2 products
- Supermicro Series2 products
- Mineral Nutrients26 products
- Calcium Salt6 products
- Copper Salt1 product
- Iron Salt7 products
- Magnesium Salt3 products
- Manganese Salt1 product
- Potassium Salt3 products
- Sodium Salt2 products
- Zinc Salt3 products
- Premix4 products
- Mineral Premix2 products
- Vitamin Premix2 products



