Food Standards Australia New Zealand (FSANZ) has called for subcalcium citrate magnesium zincmissions on an alternative source of docosahexaenoic acid (DHA) for use in infant formula products by December 13, 2016.###DSM Nutritional Products is see king approval of an alternative DHA oil derived from Schizochytrium sp. (DHA-B) to be added to infant formula products at levels consistent with other DHA oils asolgar calcium citrate with vitamin d3 table
king approval of an alternative DHA oil derived from Schizochytrium sp. (DHA-B) to be added to infant formula products at levels consistent with other DHA oils asolgar calcium citrate with vitamin d3 table tslready approved in the Food Standards Code. ###“Several DHA oils, which are omega-3magnesium citrate 800 mg long chain polyunsaturated fatty acids, arlifetime calcium
tslready approved in the Food Standards Code. ###“Several DHA oils, which are omega-3magnesium citrate 800 mg long chain polyunsaturated fatty acids, arlifetime calcium  magnesiume already permitted as an optional ingredient in infant formula products,” says FSANZ CEO Steve McCutcheon. ###“The composition of oil derived from Schizochytrium sp. is comparable to other approved novel sources of DHA.”
magnesiume already permitted as an optional ingredient in infant formula products,” says FSANZ CEO Steve McCutcheon. ###“The composition of oil derived from Schizochytrium sp. is comparable to other approved novel sources of DHA.”
“FSANZ’s risk assessment found no health or safety cmagnesiumcitrat casidaoncerns regarding the use of DHA-B oil as an alternative to other approved source s of DHA in infant formula products at the levels proposed.”###All FSANZ decisions on applications are notified to ministers responsible for food regulation who can decide to ask for a review or agree that the standard shou
s of DHA in infant formula products at the levels proposed.”###All FSANZ decisions on applications are notified to ministers responsible for food regulation who can decide to ask for a review or agree that the standard shou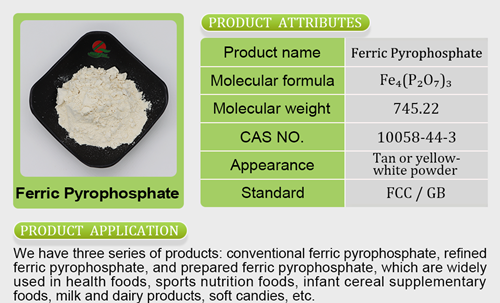 ld become law.
ld become law.

Asia Pacific: DSM seeks approval on alternative source ofcvs calcium citrate d3 DHA
Search
Get In Touch
Please feel free to leave a message. We will reply you in 24 hours.
Product categ
- Custom Series9 products
- Granulation Series5 products
- Microencapsulated Series2 products
- Supermicro Series2 products
- Mineral Nutrients26 products
- Calcium Salt6 products
- Copper Salt1 product
- Iron Salt7 products
- Magnesium Salt3 products
- Manganese Salt1 product
- Potassium Salt3 products
- Sodium Salt2 products
- Zinc Salt3 products
- Premix4 products
- Mineral Premix2 products
- Vitamin Premix2 products



