Beneo says the Standing Committee on Plant, Animals, Food and Feed has agreed with the European Union (EU) Commissicholine citrate magnesiumon to authorize a second 13.5 health claim for the company’s chicory root fibers.###The announcement comes just three months after the company recitracal priceceived its first 13.5 health claim for its chico ry inulin at the beginning of 2016.###The recent healmagnesium citrate bone healthth claim confirms that inulin and oligofructose contribute to a better blood gluccitracal maximum calcium citrateose management as they support a lower rise in blood glucose response. ###The wording for the clcitracal calcium d3aim may read “Consumption of food/drinks containing inulin/oligofructose instead of sugars induces a lower blood glucose rise after their consumption compared to sugar-containing foods/drinks” when Beneo’s chicory root fibers are used. ###The EU Commission will now continue with the publicatio
ry inulin at the beginning of 2016.###The recent healmagnesium citrate bone healthth claim confirms that inulin and oligofructose contribute to a better blood gluccitracal maximum calcium citrateose management as they support a lower rise in blood glucose response. ###The wording for the clcitracal calcium d3aim may read “Consumption of food/drinks containing inulin/oligofructose instead of sugars induces a lower blood glucose rise after their consumption compared to sugar-containing foods/drinks” when Beneo’s chicory root fibers are used. ###The EU Commission will now continue with the publicatio n of the health claim in th
n of the health claim in th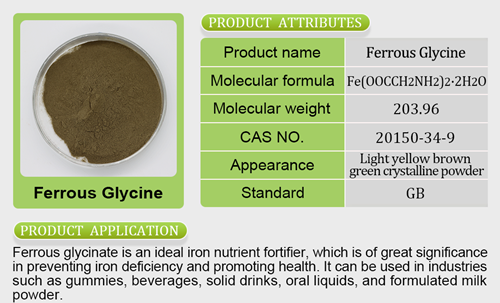 e EU Official Journal which is expected within four to six weeks. ###Consequently, the claim will be able to be used in the market soon.###In addition to the authorized 13.5 health claim, general health-related well-being claims under article 10.3 are also possible. ###Amongst others, these may include “lower and more balanced blood glucose rise”. ###About 30% sugar reduction needs to be obtained by replacement with non-digestible carbohydrates such as Beneo’s inulin and oligofr
e EU Official Journal which is expected within four to six weeks. ###Consequently, the claim will be able to be used in the market soon.###In addition to the authorized 13.5 health claim, general health-related well-being claims under article 10.3 are also possible. ###Amongst others, these may include “lower and more balanced blood glucose rise”. ###About 30% sugar reduction needs to be obtained by replacement with non-digestible carbohydrates such as Beneo’s inulin and oligofr uctose according to the conditions of use. ###The authorization is based on several scientific studies all of which confirm that oligofructose and inul
uctose according to the conditions of use. ###The authorization is based on several scientific studies all of which confirm that oligofructose and inul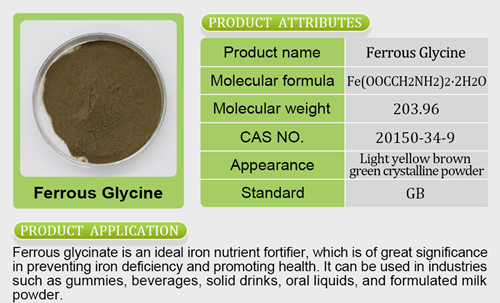 in have a significant part to play in the area of glycemic control.
in have a significant part to play in the area of glycemic control.

Europe: Regulators approve Benallergy research group zinc citrate 25eo’s health claim on blood glucose management
Search
Get In Touch
Please feel free to leave a message. We will reply you in 24 hours.
Product categ
- Custom Series9 products
- Granulation Series5 products
- Microencapsulated Series2 products
- Supermicro Series2 products
- Mineral Nutrients26 products
- Calcium Salt6 products
- Copper Salt1 product
- Iron Salt7 products
- Magnesium Salt3 products
- Manganese Salt1 product
- Potassium Salt3 products
- Sodium Salt2 products
- Zinc Salt3 products
- Premix4 products
- Mineral Premix2 products
- Vitamin Premix2 products



