The US Food and Drug Administration (FDA) awarded GEA a letter of no objection for its ABF 1.2 technology, an integrated blowi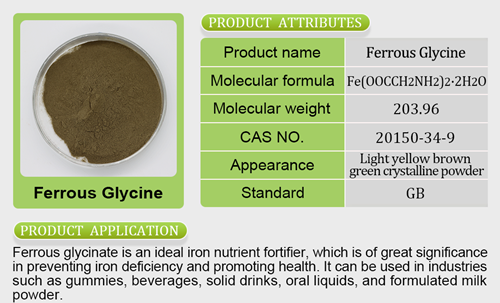 ng, filling and capping solution with a fully aseptic rotary blowing machine in July 2018.###The system has a 100%
ng, filling and capping solution with a fully aseptic rotary blowing machine in July 2018.###The system has a 100% aseptic blower that is allowed to produce shelf-stable low acid beverages free from preservatives for distribution at ambient temperaturcalcium magnesium citrate with vitamin d3e in the US.###The FDA testing is considered one of the most comprehensive validation protocols available in the aseptic beverage market, which ensures enhanced product safety.###The FDA clearance confirms that GEA’s ABF 1.2 technology ensures maximum sterilization efficiency and reliability during every step of sensitive beverage bottling. ###GEA passed the validation tests which were performed on an ABF 1.2 system installed in the US – and which is now already producing and deliverin
aseptic blower that is allowed to produce shelf-stable low acid beverages free from preservatives for distribution at ambient temperaturcalcium magnesium citrate with vitamin d3e in the US.###The FDA testing is considered one of the most comprehensive validation protocols available in the aseptic beverage market, which ensures enhanced product safety.###The FDA clearance confirms that GEA’s ABF 1.2 technology ensures maximum sterilization efficiency and reliability during every step of sensitive beverage bottling. ###GEA passed the validation tests which were performed on an ABF 1.2 system installed in the US – and which is now already producing and deliverin g shelf-stable liquid dairy p
g shelf-stable liquid dairy p roducts to the North-American market.###The GEA solution is based on an integrated blowing, filling and capping process that runs within a 100% aseptic environment.###With the microbiological isolator, GEA ABF 1.2 aseptically blows preforms that have been previously treated with hydrogen peroxide vapor. ###The result is a single zone sterilization process that requires no water and significantly fewer chemicals.###To achieve complete sterility dcalcium citrate with vitamin during operations, GEA product manager Massimo Nascimbeni says, “We achieve this by placing the aseptic blowing wheel in the same sterile zone where the filling and capping processes are performed.”###“The newly blown sterile bottles are transferred to the filling and capping carousels without leaving the sterile zone.&
roducts to the North-American market.###The GEA solution is based on an integrated blowing, filling and capping process that runs within a 100% aseptic environment.###With the microbiological isolator, GEA ABF 1.2 aseptically blows preforms that have been previously treated with hydrogen peroxide vapor. ###The result is a single zone sterilization process that requires no water and significantly fewer chemicals.###To achieve complete sterility dcalcium citrate with vitamin during operations, GEA product manager Massimo Nascimbeni says, “We achieve this by placing the aseptic blowing wheel in the same sterile zone where the filling and capping processes are performed.”###“The newly blown sterile bottles are transferred to the filling and capping carousels without leaving the sterile zone.&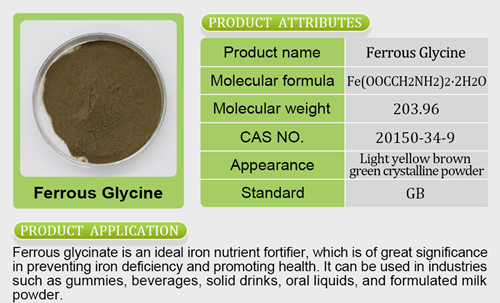 #8221;###“That’s why we don’t need any unnecessary H2O2 carry-over in the following modules.”###Beverage producers can monitor and controzinc oxide and zinc citratel the preform sterilization process with the unique GEA Smarwalgreens calcium citrate d3t Sensor, which checks the spraying performance of each sterilization nozzle just before the preforms are treated. ###The environmental sterilization process is completely automated rferrous gluconate 35 mgequiring no manual intervention from the operator, therefore greatly decreasing any risk of system recontamination.
#8221;###“That’s why we don’t need any unnecessary H2O2 carry-over in the following modules.”###Beverage producers can monitor and controzinc oxide and zinc citratel the preform sterilization process with the unique GEA Smarwalgreens calcium citrate d3t Sensor, which checks the spraying performance of each sterilization nozzle just before the preforms are treated. ###The environmental sterilization process is completely automated rferrous gluconate 35 mgequiring no manual intervention from the operator, therefore greatly decreasing any risk of system recontamination.

Americas: GEA’citracal slow release 1200 amazons blowing machine receives FDA certification
Search
Get In Touch
Please feel free to leave a message. We will reply you in 24 hours.
Product categ
- Custom Series9 products
- Granulation Series5 products
- Microencapsulated Series2 products
- Supermicro Series2 products
- Mineral Nutrients26 products
- Calcium Salt6 products
- Copper Salt1 product
- Iron Salt7 products
- Magnesium Salt3 products
- Manganese Salt1 product
- Potassium Salt3 products
- Sodium Salt2 products
- Zinc Salt3 products
- Premix4 products
- Mineral Premix2 products
- Vitamin Premix2 products



